Following implantation, the developing human zygote becomes an embryo and the formation of structures that eventually form organs and organ systems begins. The cells of the inner cell mass of the blastocyst, the embryonic stem cells, have the capacity to develop into every type of cell in the body. Scientists refer to them as pluripotent cells. Embryology is the study of how these pluripotent cells change or differentiate into specialized cells of organ systems. The amazing processes of morphogenesis are extremely complex and highly orchestrated. A multitude of genes are switched on and off; proteins and growth factors are produced and interact with cells to change their behavior; cells move and migrate to different locations in the growing embryo under the influence of mechanical forces; cell patterns for organ formation and body orientation are laid down. Some cells may live for a short time and then die off by apoptosis, one form of programmed cell death. All these intricate actions are vital for the remodeling of the structures formed at the various stages of embryo development. If this cellular choreography is altered in some way, such as the absence of a gene, abnormalities in development occurs leading to congenital anomalies in the newborn. Environmental factors such as air pollution or contaminated drinking water or a multitude of chemical pollutions can alter embryogenesis as well. A classic example is excessive maternal alcohol consumption leading to fetal alcohol syndrome where the newborn has small eyes, flat lips and a fattened groove under the nose, low body weight, learning disabilities, developmental delay and hyperactive behavior.
The development of the embryo is a challenge to understand. It helps to visualize the events that occur as a dynamic action movie. Embryology is a visual science. For this reason, we include a link to an open-source video describing embryogenesis here. It would be helpful to you, the reader of this blog, to view it now before reading further.
Right after implantation, the embryo starts to develop three distinct cell layers that are precursor cells to the organs that arise from each distinct layer. These are named, the endoderm, mesoderm, and ectoderm. This stage of development is called gastrulation and starts fourteen days after fertilization and ends approximately one week later. Remember that the cells of the endoderm will eventually differentiate to form the gastrointestinal tract, the respiratory system, thymus, parathyroid glands, bladder, and urethra, while the cells of the mesoderm develop into the circulatory system and heart, blood and lymphatic system, ureters, and adrenal cortex. The ectoderm is the precursor for the skin, nervous system, and a part of the sensory system. These cells are multipotent, meaning that they can differentiate into any cell that will be developed in their specific cell layer. During gastrulation the embryo also forms the orientation of the developing human embryo into a cephalic (head) area, caudal (tail) area and back and front. The organization of this orientation is regulated by HOX genes. Oocytes and sperm develop from specialized cells, primordial germ cells, set aside in the yolk sac outside the embryo itself. Prior to gastrulation they migrate to an area of the embryo called the genital ridge. The cells of the genital ridge eventually develop into either an ovary or testis. Initially, embryos whether they have XY chromosomes or XX chromosomes have two precursor reproductive ducts, the Wolffian duct and the Mullerian duct. In the male embryo the Mullerian duct is lost allowing for the male reproductive tract to develop from the Wolffian duct. In the female embryo the Wolffian duct degenerates and the Mullerian duct develops into the female reproductive tract. The nervous system develops by a process called neuralation that occurs just after gastrulation. During this process the primitive notochord closes and becomes the neural tube at about week four. From week five to eight the central nervous system develops with the brain developing at the cephalic area of the embryo. By week seven the embryo has obtained a characteristic C shape and has developed upper and lower limb buds.
Since this short blog is unable to present in some detail how all the organs form, we will discuss the development of the cardiac system as one example of the complexity of the process. There are several excellent and well documented presentations of embryology online if you would like to learn more details.
They are:
- https://bhsc.queensu.ca/courses/anat-471-human-embryology
- https://www.unmc.edu/genetics/education/summer-program/medical-embryology.html
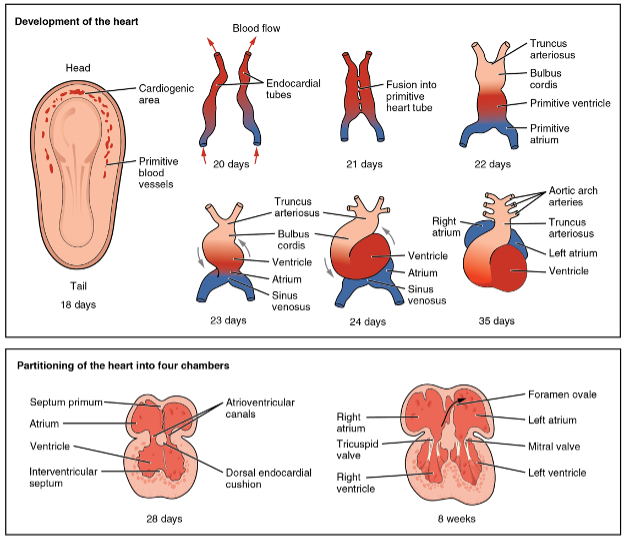
The heart develops from the embryonic mesoderm. On either side of the embryo in the cephalic area of the embryo a cardiogenic area forms at day 18 and by day 20 two endocardial tubes form on either side. The tubes then move together at day 21 to form the primitive heart tube. This tube then develops five distinct areas. By day 23 this tubular heart begins to rotate or “loop” to form heart chambers. By 8 weeks the heart has four separate chambers and values, well-illustrated by the open-source diagram below.
The cardiac myocytes in the endocardial tube spontaneously begin to contract triggered by electrical depolarization at 21 days after fertilization (five weeks after the maternal last menstrual period). This electrical activity can be noted on ultrasound and can be picked up by doppler transducers. The heart is sufficiently developed to have a functional heart beat at 17 to 20 weeks gestational age. At that stage the cardiac myocytes have formed a syncytium whereby the electrical activity is coordinated. As the blood cells have formed very early in embryogenesis blood is circulated though the primitive endocardial tubes. It is important to appreciate that the blood circulation in the embryo and later the fetus is very different than that of the neonate since the fetal lungs are not yet breathing in oxygen and expiring carbon dioxide.
Since it is difficult to study human embryology, it is appropriate to ask, how is it the scientists know these facts. In the early part of the 20th century several scientists in the United States as well as Europe began to collect specimens of embryos from spontaneous abortions (miscarriages) ectopic pregnancies, embryos found in hysterectomy specimens performed for fibroid tumors (in the days when pregnancy tests were not available) and from autopsy material from young women. In the United States, the Carnegie Collection was started in 1914 and has painstakingly collected over 800 specimens for study, This collection is housed in the Carnegie Institution of Washington Department of Embryology and today includes samples from many other collections. This collection is still used by scientists today. There are collections in Europe as well. One such source of ethically derived embryos is the Human Developmental Biology Resource.
Mouse models are commonly used in developmental biology as the embryogenesis is these animals is very similar to that of humans. There are differences however. The early mouse embryo is cylindrical while the human early embryo is shaped like a disc. A very recent and very careful study of the transcriptome of a 16-19 fertilization day embryo using single cell RNA seq methods has shown that compared to the mouse, the human embryo develops the hematopoietic (blood) cells at an earlier stage of gastrulation. This study was conducted at Oxford University by Richard Tyser and colleagues.
Embryological studies are important to medical science as they show how congenital defects occur. In 2022, The Campion Fund gave an award to Ciro Amato, PhD of the National Institute of Environmental Health Sciences’ Laboratory of Reproduction and Developmental Biology for his work in the discovery of a novel group of cells in the embryo that are important in the closure of the male urethra. Abnormalities in the development of the male urethra leads to the condition of hypospadias where the male urethra opening occurs in the penile shaft rather than at the tip of the penis. This can be a devasting anomaly for the male newborn often needing surgical correction. This new information can be used to develop prevention and therapies for hypospadias in the future. By studying how the neural tube closes in the embryo medical science learned how to prevent spina bifida and encephalocele. Today by giving folic acid before and during pregnancy these serious defects can be prevented.
Selected Bibliography
Crispi F, Sepúlveda-Martínez Á, Crovetto F, Gómez O, Bijnens B, Gratacós E. Main Patterns of Fetal Cardiac Remodeling. Fetal Diagn Ther. 2020;47(5):337-344. doi: 10.1159/000506047. Epub 2020 Mar 26. PMID: 32213773.
Gasser RF, Cork RJ, Stillwell BJ, McWilliams DT. Rebirth of human embryology. Dev Dyn. 2014 May;243(5):621-8. doi: 10.1002/dvdy.24110. Epub 2014 Feb 27. PMID: 24395627; PMCID: PMC4310677
Hikspoors, J.P.J.M., Kruepunga, N., Mommen, G.M.C. et al. A pictorial account of the human embryonic heart between 3.5 and 8 weeks of development. Commun Biol 5, 226 (2022). https://doi.org/10.1038/s42003-022-03153-x
https://www.youtube.com/watch?v=dgPCDXmcQjM
Illustration from Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013.
Tan CMJ, Lewandowski AJ. The Transitional Heart: From Early Embryonic and Fetal Development to Neonatal Life. Fetal Diagn Ther. 2020;47(5):373-386. doi: 10.1159/000501906. Epub 2019 Sep 18. PMID: 31533099; PMCID: PMC7265763.
Tyser, R.C.V., Mahammadov, E., Nakanoh, S. et al. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature 600, 285–289 (2021). https://doi.org/10.1038/s41586-021-04158-y
Wellner, Karen, "Carnegie Institution of Washington Department of Embryology". Embryo Project Encyclopedia (2010-06-27). ISSN: 1940-5030 http://embryo.asu.edu/handle/10776/1677.