Introduction
Implantation and decidualization are extremely important events in early pregnancy when the embryo and trophoblast cells embed in the endometrium of the uterus. The trophoblast becomes the placenta and will nourish the fetus to ensure healthy growth and development throughout the entire pregnancy. Implantation and decidualization processes occur by an intricate and highly coordinated interaction, what scientists call “cross-talk” between the blastocyst and the receptive endometrium. Alterations in these coordinated physiological events will lead to poor pregnancy and infant outcomes.
There is an extremely important fact to appreciate in understanding the whole process of implantation. There are two ways to describe the length of human pregnancy. One is the concept of fertilization age. This dates a pregnancy from the time of fertilization. The other is the concept of gestational age. This dates a pregnancy from the date of the last menstrual period. It is a fact that fertilization occurs about 14 days or two weeks after the last menstrual period. Thus, in using gestational age as a term, one must always realize that there is an average two-week time period when there was no new organism. Thus, when someone says a pregnancy is 6 weeks gestational age, they are really talking about a four-week-old embryo. Fifteen weeks of gestational age is thirteen weeks fertilization age and the fetus is only thirteen weeks old. Why do we use both terms, today? It is confusing for most people, but not for professionals in the field. How did this happen? Consider that for all the eons of human life on earth there have always been individuals who have attended births to help mother and baby. Birth attendants long ago did not have the scientific understanding we have today, but they were intelligent. They were very observant and over time the connection between the cessation of menstrual periods and pregnancy was made. Information of when the last menstrual period occurred was used to predict the timing of birth more or less. This idea became established and as midwifery and medicine advanced the concept of dating pregnancy from the last menstrual period persisted. Clinical information and data accumulated. Modern and up to date obstetrical knowledge is built on this past data. It is necessary to keep this gestational age concept in order not to lose past knowledge that has accumulated. Today all scientists, physicians, midwives, nurses know and accept the difference between gestational age and fertilization age even though they may use one or the other as their reference point.
It is helpful to appreciate that implantation is a very dynamic process, as are all other events of pregnancy and the development of the zygote to embryo and fetus. Nothing is static. Imagine a movie not still pictures. Cells grow and divide, and change shape. They move around or migrate to different anatomic positions throughout these events. The timeframe of these events is mentioned in our blogs to provide a sense of the dynamic actions. It can be confusing as the names of cells change as they are changed or in the language of science, transformed.
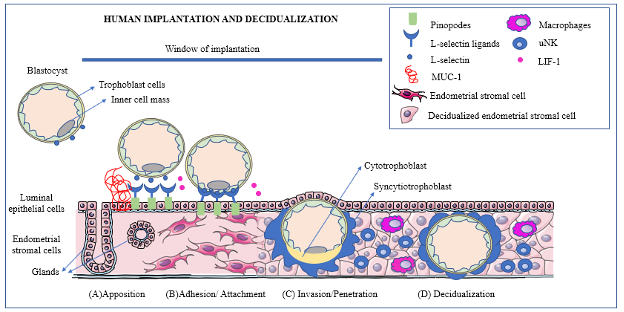
Pregnancy in humans is established by two important physiological events that occur simultaneously: implantation of the blastocyst which will become an embryo, into the uterus and decidualization, or changes in the endometrium that are necessary to sustain a pregnancy. These two processes are critical for the establishment of the interactions between mother and developing embryo. First the blastocyst which is free in the uterine cavity needs to line up in a proper order with the endometrium: a step called apposition. Only then does the adhesion/attachment step occur. The next event is the invasion of the blastocyst into the endometrium and eventually into the uterine endometrial stroma to contact the maternal blood vessels. The changes of the endometrium called decidualization are then finalized. Finally, there is a process of regulation of the immune system that will prevent the mother’s immune responses from rejecting the zygote which is a totally different organism. The entire processes of implantation including decidualization occur five to seven days after fertilization and last about three days. They must occur during a time when the endometrium is primed or ready for implantation. This time is called the “window of Implantation” by scientists and physicians.
Glossary:
Adhesion: is the second phase of implantation when the blastocyst attaches to the endometrium.
Apposition: is the first phase of implantation when the blastocyst and the endometrium become aligned and in proper position for implantation.
Blastocyst: is composed of cells of the inner cell mass and a thin outer cellular layer. It is a ball with a hallow center. The blastocyst may contain as many as 200 to 300 cells formed after 32 cell divisions. Some authorities say the blastocyst contains 70-100 cells. The blastocyst contacts the endometrium during implantation.
Chorionic gonadotropin: A hormone produced by the trophoblast cells. It is detected in maternal blood around ten days after fertilization.
Corpus Luteum of Pregnancy: This is a structure in the ovary that produces progesterone, a hormone that maintains pregnancy. It forms in the ovary after ovulation and is a temporary structure and breaks down when it is no longer needed.
Cytokines: are small signaling proteins that bind to specific receptors on cells to exert their functions. They regulate immune responses and modulate growth and maturation of cells:
Cytotrophoblast: is the name of the inner layer of trophoblast cells.
Decidualization: is the process of transforming endometrial stroma cells which are elongated fibroblast-like cells into decidual cells which are round epithelial-like cells. In humans decidualization begins during the menstrual cycle at the mid-secretory phase and continues during implantation.
Embryo: The zygote becomes an embryo at the time of implantation and remains an embryo until it becomes a fetus at ten weeks gestational age or eight weeks after fertilization.
Fertilization Age: This is the age of a zygote-embryo-fetus calculated from the time of fertilization.
Gestational Age: The age of a pregnancy calculated from the first day of the last menstrual period. This dating of pregnancy is used by clinicians.
Implantation: Occurs when the blastocyst embeds in the endometrium. Implantation occurs about 5-7 days after fertilization.
Inner Cell Mass: is the area of the blastocyst three to four cells thick that will develop into the embryo and then the fetus.
Invasion: is the phase of implantation when the trophoblast penetrates the endometrium. During this phase the trophoblast cells contact the maternal spiral arteries.
LIF (Leukemia Inhibitory Factor): This cytokine belonging to the Interleukin-6 family is secreted by the luminal epithelium of the endometrium. It regulates trophoblast cell adhesion playing an important role during attachment of the blastocyst to the pinopodes.
MUC-1: This is a heavily glycosylated transmembrane glycoprotein expressed at the tip or apical surface of the endometrial epithelium. It prevents cell degradation and is dependent on progesterone for its expression. It is removed from the endometrium as the result of signals from the blastocyst at the time of implantation at the beginning of the adhesion phase.
Pinopodes: These are protrusions of the tip of the uterine epithelial cells which appear at day 19-21 of gestation or 5-7 days after fertilization. They are associated with LIF expression.
Selectins: are glycoproteins with a transmembrane domain (or part) and a small tail. L-Selectin is present on the surface of the blastocyst and takes part in the adhesion to the endometrial epithelium.
Spiral arteries: Maternal blood vessels in the endometrium. The trophoblast cells invade these arteries at the start of the process of formation of the placenta.
Syncytiotrophoblast: This is the outer layer of trophoblast cells that form a barrier between the maternal and fetal blood and allows passage of nutrients and waste products. It also functions as endocrine tissue secreting hormones, estrogen, progesterone, and chorionic gonadotropin.
Trophoblast: is the outer layer of the blastocyst that contacts the endometrium during implantation.
Uterine NK cells: are special immune cells in the decidua that release growth factors and cytokines to assist in the control of blastocyst invasion. The play an important role in maternal immune tolerance.
Window of implantation: occurs at gestational age 19-21 or fertilization age of 5-7 days and is the time when implantation happens.
Zonal Hatching: is the process when the zona pellucida is degraded allowing for adhesion of the blastocyst to the endometrium.
Zygote: is the diploid cell that forms with the union of two haploid cells, the sperm and ovum. It divides and develops into and embryo.
The Zygote Moves Through the Fallopian Tube to the Uterus
The fertilized ovum, properly called a zygote moves toward the uterus by the motion of the cilia along the lumen of the Fallopian Tube. During this journey cell division occurs. The zygote divides into two cells, then four, then eight and then sixteen cells. At this stage it is called a morula. It is a ball of compacted cells, still surrounded by the zona pellucida described in the previous blog on fertilization. When the zygote arrives in the upper uterine cavity it becomes a blastocyst during the apposition phase. The blastocyst is a ball of cells with a hollow center. Around the outside is a layer one cell thick. There is one area that is three to four cells thick. This area, called the inner call mass will develop into the embryo and then the fetus. The outer layer is called the trophoblast and will become the fetal membranes and placenta.. For the blastocyst to implant into the endometrium it must lose the zona pellucida. The process by which this occurs is called zona hatching. The blastocyst secretes enzymes that cause the zona pellucida to disintegrate. Now the blastocyst and the endometrial are ready for apposition where they lineup together in a precise way.
Endometrial Window of Implantation
Just as the blastocyst prepares for the process of implantation, so does the uterine endometrium, the inner layer of the uterus lining the uterine cavity. This layer contains epithelial cells, immune cells, endothelial cells, and stromal cells. The window of implantation is the time when the endometrium can receive the blastocyst. It occurs in humans during the phase of the menstrual cycle called the secretory phase, a time when progesterone is the predominate hormone following ovulation about day 20-24 of the menstrual cycle and lasts about 24-36 hours. Pinopodes are microvilli which are mushroom- like protrusions of the apical cell membranes (or tip of the cells) of the uterine epithelial cells in the endometrium. They appear at gestational age 19-21 days (fertilization days 5-7). Pinopodes formation is enhanced by progesterone. Pinopodes are associated with increased leukemia inhibitory factor or LIF and receptors of LIF, progesterone and an adhesion molecule called integrin αVβ3. All of these are involved in the communication between the endometrium and the blastocyst. Mucin-1 or MUC-1, a transmembrane glycoprotein is expressed on the apical surface or tip of the epithelial cells and increases during the window of implantation. It functions by protecting enzymes from degrading the cells. MUC-1 acts as a barrier for implantation of the blastocyst until removal during adhesion. Interleukine-6 (IL-6) is produced in the endometrial and stromal cells during implantation. It is also secreted by the blastocyst and is a mediator of the implantation process. Another molecule is Interleukin-1 that modulates the communication between the blastocyst and the endometrium. There are many other mediators of implantation that are not mentioned in this blot. The listed references provide information regarding their roles.
Apposition:
Just as it takes the effort of both the crew and captain of a large ocean- going ship and accompanying tugboat crew as well as workers on shore to dock the ship properly, it takes the orchestrated program of many secrted molecules to set up the contact between the blastocyst and the endometrium. At first the blastocyst rolls over the endometrium. This contact occurs when the zona pellucida has broken down enough for the blastocyst to make direct contact with the endometrium. This apposition phase causes a very loose interaction between the blastocyst and the endometrium. While rolling over the endometrium, the blastocyst produces an adhesion molecule, called L-Selectin. This molecule ensures the rolling motion of the blastocyst and then tethering of the blastocyst to the endometrium in a specific alignment, as the inner cell mass must be positioned properly. The pinopodes have specific molecules called L-Selectin Ligands that initiate blastocyst adhesion. The anti-adhesion molecule MUC-1 at this apposition stage prevents the blastocyst from landing in an area of the endometrium where the chance for further implantation is poor. Interaction between the blastocyst and the endometrium occurs by an intricate and highly coordinated signaling pattern, what scientists call “cross-talk”. If there are alterations in coordination of this interaction, implantation is impaired or may not actually happen.
Adhesion/Attachment:
At the beginning of this phase, the breakdown of MUC-1 is initiated by the blastocyst to allow adhesion. Cytokines and chemokines are induced. They function by attracting the blastocyst to the location of implantation. One of the most important molecules is the LIF on the pinopodes. In humans, deficiency of LIF is associated clinically with infertility. Integrin αVβ3, expressed by trophoblast cells and the uterine epithelium, is important for recognition of the endometrium during this phase of implantation also. When this integrin is abnormal, an association with recurrent pregnancy loss is found.
Invasion:
During this phase the trophoblast cells penetrate and invade the endometrial stroma. They develop thin folds or invadopodia that grow in between the endometrial epithelial cells. The cells then degrade the basement membrane of the epithelium and spread into the stroma. At this point the trophoblast cells grow and become either the inner cytotrophoblasts or the syncytiotrophoblasts. The syncotiotrophblast cells produce chorionic gonadotropin or HCG. This hormone is detected in maternal blood ten days after fertilization and is used clinically as a marker for pregnancy and pregnancy viability. HCG promotes the production of progesterone by the corpus luteum which is the area where the secondary oocyte broke out of the ovary. This corpus luteum has been producing progesterone and its continued production is assured by the action of HCG. Progesterone is a vital hormone for the maintenance of pregnancy as it suppresses uterine muscle contractions, promotes the development of blood vessels in the myometrium, interacts with immune cells ensuring that the embryo is not rejected by the maternal immune system and modulates T cells of the immune system during implantation. The inner cell mass of the blastocyst follows along with the embedding trophoblast. The whole process of implantation is complete by day 8 of the fertilization age. The entry site is then covered with fibrin. The syncytiotrophoblasts have fluid filled spaces which are separated by trabeculae that are arranged radially in a circle to form a primary chorionic villus. Mutliple primary villi branch into secondary and tertiary villi as time passes to form the placenta. By 9 days after fertilization the cells break through the maternal spiral arteries and maternal veins at the start of placental development. Implantation is complete on fertilization day 10. At this point in time the blastocyst becomes an embryo.
Decidualization:
The endometrial stroma cells (elongated fibroblast-like cells) are transformed into decidual cells (round epithelial-like cells) during this process. In humans the transformation to decidual cells starts during the menstrual cycle at the mid-secretory phase and is under the control of progesterone. If pregnancy does not happen, menses will eventually occur at 28 to 32 days following the last period. When a pregnancy occurs, elevated levels of progesterone continue and work to maintain the decidual cells. During the decidualization event the endometrium acquires an increase in vascular permeability along with an invasion of white blood cells (leukocytes). New blood vessels are formed and grow. Other cells such as macrophages, lymphocytes are involved in decidualization as well. Special cells, call the uterine natural killer cells or NK cells infiltrate the decidua and release growth factors and cytokines that assist in the control of the invasion of the blastocyst. Seventy percent of the decidual immune cells are uterine natural killer cells. These cells are highly involved in the establishment of the maternal immune tolerance so important to the maintenance of a pregnancy by preventing maternal rejection of the implanted embryo and later the fetus. (NK cells are part of the innate immune system and are found in other parts of the body where they respond to anything that cells sense as “non-self”. They play a vital role in tissue by destroying infected and/or diseased cells such as cancer cells.) Progesterone also regulates the Notch pathway, a signaling pathway that is important in tissue morphogenesis. This signaling pathway is an important contributor to the transformation of stromal fibroblasts into decidual cells.
Alterations of Implantation:
Recurrent miscarriage or spontaneous pregnancy loss is defined as the loss of two or more pregnancies. Many factors such as interference with the formation of new blood vessels during decidualization, an imbalance of the immune function, such as increased accumulation of uterine NK cells, and/or dysregulation of cytokine production, will interfere with successful implantation causing recurrent miscarriage. Preeclampsia, a condition in pregnancy where the maternal blood pressure is seriously elevated is associated with poor or “shallow” invasion by the trophoblast cells as well as an imbalance in cytokine production by the uterine nature killer cells. Maternal disease conditions like endometriosis, uterine fibroids interfere with implantation.
Bibliography for further reading:
Huang CC, Hsueh YW, Chang CW, Hsu HC, Yang TC, Lin WC, Chang HM. Establishment of the fetal-maternal interface: developmental events in human implantation and placentation. Front Cell Dev Biol. 2023 May 17;11:1200330. doi: 10.3389/fcell.2023.1200330. PMID: 37266451; PMCID: PMC10230101.
Muter J, Lynch VJ, McCoy RC, Brosens JJ. Human embryo implantation. Development. 2023 May 15;150(10):dev201507. doi: 10.1242/dev.201507. Epub 2023 May 31. PMID: 37254877; PMCID: PMC10281521.
Ochoa-Bernal MA, Fazleabas AT. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int J Mol Sci. 2020 Mar 13;21(6):1973. doi: 10.3390/ijms21061973. PMID: 32183093; PMCID: PMC7139778.
Sojka DK, Yang L, Yokoyama WM. Uterine Natural Killer Cells. Front Immunol. 2019 May 1;10:960. doi: 10.3389/fimmu.2019.00960. PMID: 31118936; PMCID: PMC6504766.